%2016_9.png)
Welcome to Investors’ Edge - your daily dose of business insights, trends, and updates that matter. In this space, we go beyond the headlines to explore the evolving world of companies and industries. Here we bring you thoughtfully curated insights, sharp observations, and key developments shaping the business landscape.
Whether it's a strategic pivot by a market leader or an under-the-radar company making waves, we break it down for you — clearly, concisely, and consistently.
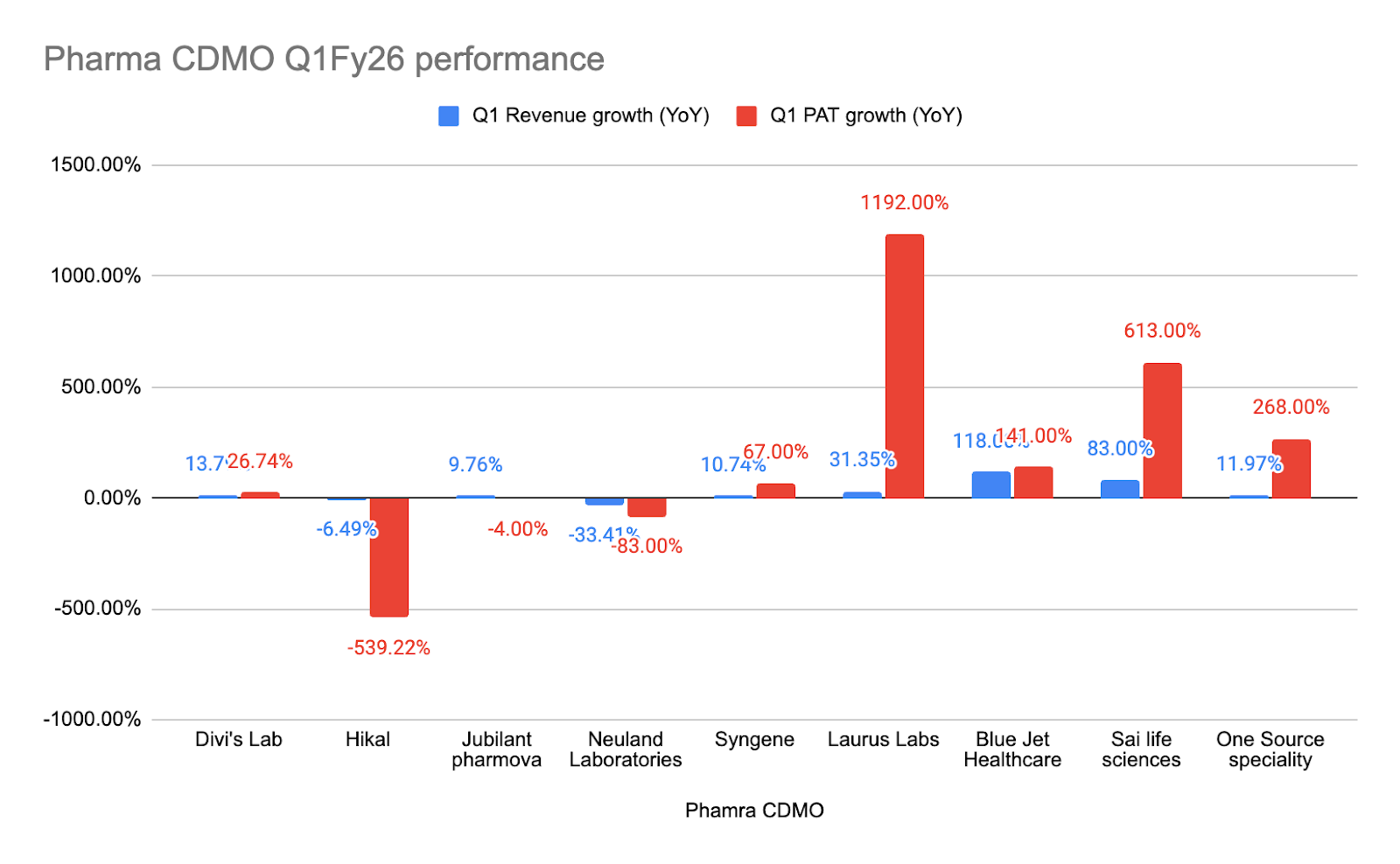
Welcome to the 22nd edition of Investor's Edge! In this issue, we delve into the Q1FY26 results and concalls of prominent Pharma CDMO players. We'll uncover the reasons behind both the underperformance and outperformance observed across all the companies we cover today. Let's begin!
The Q1 FY26 earnings calls and presentations from leading Indian pharmaceutical CDMO and API companies reveal a sector navigating a complex landscape of global demand shifts, pricing pressures, regulatory scrutiny, and strategic investments for long-term growth.
While some companies experienced a muted start to the fiscal year, primarily due to deferred shipments or inventory adjustments, a strong underlying optimism for future growth prevails, driven by "China Plus One" strategies, increasing outsourcing by global innovators, and significant investments in advanced capabilities and capacity expansion, particularly in high-growth modalities like peptides and biologics.
Many companies reported a slow Q1 FY26, attributing it to lumpiness inherent in the CDMO business, deferred shipments, or inventory adjustments. Despite this, management teams largely maintained their full-year guidance, anticipating a stronger H2 FY26.
Neuland Laboratories
"FY25 was a year of consolidation and Q1FY26 has been a continuation of that, our customer pipeline gives us good visibility. Hence, we are confident with the commercialization of Unit 3 production block. It will give the business greater revenue momentum from the latter half of FY26."
- Abhijit Majumdar, CFO
Commercialisation of molecules to begin from H2FY26
“I think we kind of carefully crafted the opening remarks to give as much detail and as much guidance to investors about how we see FY26 unfolding and we have kind of tried Neuland Laboratories Limited July 31, 2025 Page 7 of 17 to lay everything out there. So I think you have kind of caught most of the points that were said. We really don't wish to break things down at a quarterly level because that is something that we have avoided doing in the past and we do not want to set a new precedent in terms of trying to break things down. But we do remain very clear and confident that you will have that growth that we had indicated in FY26. Yes, the validation of our Unit 3 facility for the CMS molecule is complete and therefore, anticipation of commercial supplies is one milestone, as you have clearly acknowledged. I think the launch of the new CMS molecule, which was awaited for about a year or so, is another milestone, which we have indicated. And there has been another CMS drug, which was approved last year, which we are also scaling up. I think these are some of the critical milestones. There are a few on the GDS side. But we would unfortunately not be able to give you a timing indication. You should be able to see progress during the course of the year.”
Jubilant Pharmova
Revenue grew by 10% YoY to INR 1,901 Cr. EBITDA grew by 14% to INR 302 Cr. Normalized PAT increased by 48% to INR 103 Cr. The company is "On track towards Vision 2030" with "Solid growth momentum along with EBITDA & PAT margin expansion."
Laurus Labs
"Strong performance continued in Q1; ₹ 1,570 Cr Revenues and 31% revenues growth." Gross margins remained strong at 59.4%.
Healthy pipeline of molecules
Pipeline momentum was healthy across the clinical commercial phase with the mix shifting towards increased Big Pharma projects. In specific, a lot of customer interest seen around biocatalysis, flow chemistry, high-energy chemistry, continuous manufacturing, peptide manufacturing, etc. As on date, we have a pipeline of over 110 active projects, over 90 in Human Health and about 20 in Animal Health and Crop Sciences.
Blue Jet Healthcare
Revenue from operations was up 118% YoY, reaching INR 3,548 million. However, EBITDA margins saw a QoQ decline due to product mix and inventory adjustments. "We remain confident in our outlook for FY'26. Demand visibility across key customers is healthy. Capacity is now in place and product pipelines are expanding."
- Shiven Arora, Managing Directo
Hikal Limited
Reported consolidated revenue of INR 380 crores and EBITDA of INR 25 crores, attributing the impact to "deferment of shipments in our Pharmaceutical business." The company "remains confident of delivering on our yearly guidance... We expect performance to improve meaningfully in the second half of the financial year with Q4 being the strongest quarter of the year."
- Sameer Hiremath, Managing Director
OneSource Specialty Pharma
"our Q1 performance has been in line with our expectations... our first half of the year as you know primarily we'll be focusing as Arun just mentioned on executing our MSAs while the second half will be driven by the commercial supplies of Samaglutide."
“Our Q1 performance has been in line with our expectations. During the quarter, we secured 6 new contracts and received 25 RFPs across all our offerings. In preparation for forthcoming DDC commercial launches, we have undertaken major capacity expansion work in our flagship site. Our unrelenting focus on quality and compliance was once again validated with our flagship site successfully clearing back-to-back inspections from USFDA and ANVISA Brazil paving the way for commercialisation.”
- Neeraj Sharma, CEO & MD
Sai Life Sciences
"We have begun FY26 on a strong footing, delivering healthy performance across our Discovery, Development and Commercial Manufacturing businesses." Total revenue up 77% YoY, led by 113% growth in CDMO and 38% rise in Discovery. EBITDA grew 305% YoY with margins expanding to 25%.
Divi's Laboratories
Reported a 14% constant currency growth for the quarter. Product mix for generics to custom synthesis was 47% and 53% respectively. "The generics business had a higher component in this quarter compared to the custom synthesis. However, we have always maintained the stand that there could be lumpiness in one particular quarter with respect to generics and in another quarter with respect to custom synthesis."
- Nilima Motaparti, Whole-Time Director, Commercial
Syngene International
Reported revenues up 11% YoY (7% in constant currency). Operating EBITDA margin improved to 23.6%. "We are pleased with the performance in the first quarter, which is both in line with our expectations and establishes a good trajectory for the rest of the financial year."
- Peter Bains, Managing Director and CEO
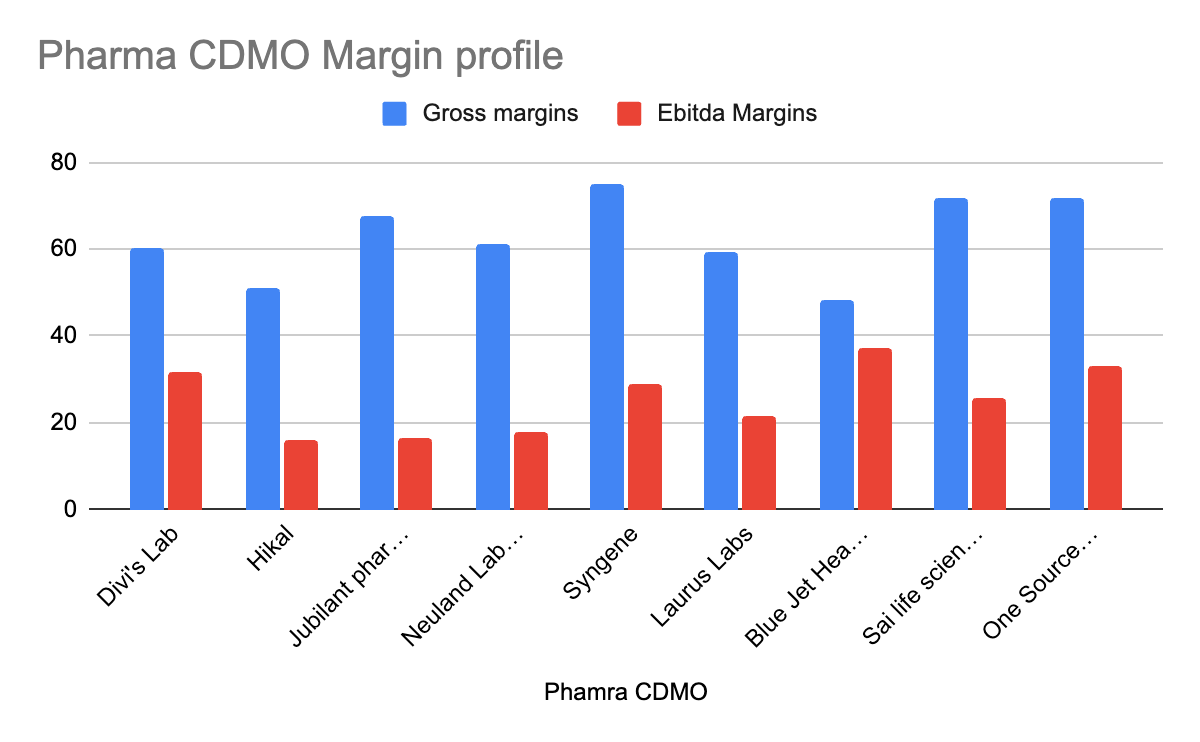
Gross margins generally remained strong or saw slight fluctuations due to product mix or temporary inventory effects. EBITDA margins were impacted by lower revenue in some cases, leading to deleveraging of operating expenses, but are expected to recover as sales volumes pick up.
Neuland Laboratories
EBITDA margin at 14.4%. "The decreased revenue has led to impact on margins as a result of deleverage, and there has been no other significant factor impacting the profit and loss account." - Abhijit Majumdar, CFO. Saharsh Davuluri added, "we see margins also recovering because the margins have been suppressed because of the poor sales."
Laurus Labs
Product mix change over upcoming 5 years
Gross margins at 59.4%, up 430 bps QoQ. EBITDA margins at 24.8%, up 1050 bps YoY.
“While we continue to focus on our core, which is APIs and integrated offerings in generics, we are also increasing our investments, capex, resources and technology platforms to offer a wide variety of services for late clinical and commercial Human health, Animal health and Crop science programs. As we've mentioned, currently, the CDMO contributes over 30% of our revenue. We expect it will continue to grow. In the near to medium term future, we expect it has the potential to touch 50%. That's our guesstimate
Jubilant pharmova
"Strong customer traction in CDMO Sterile Injectable business." Line 3 in Spokane seeing "excellent traction in Requests for Proposals (RFPs) for Line 3 including from Big Pharma. We expect to finalise these within FY26."
Blue Jet Healthcare
Gross margin at 48.5%, down from 55% QoQ, primarily due to inventory adjustment and product mix. "We don't give guidance on the future. So, based on the current portfolio mix, it's going to be 53 and we will be in a position to sustain similar levels in the coming quarters."
- Ganesh Karuppannan, CFO
Hikal Limited
EBITDA margin stood at 6.5%, down from 14.3% in Q1 FY25, attributed to "under-absorption of fixed costs, less favorable product mix and lower capacity utilization."
- Kuldeep Jain, CFO
Sai Life Sciences
EBITDA margin expanded by 14% YoY to 25%. "We believe that this business can be scaled to achieve 28 to 30% margin as our revenues grow and our results for this quarter show a positive movement towards that goal."
- Siva Chittor, CFO
Syngene International:
Operating EBITDA margin at 23.6% (vs 21.5% in Q1 FY25). "We expect EBITDA margins to be in the mid-20s for the full year."
“Okay. So, in our small molecule’s facility, I think you've understood well my comment that the versatility of the facility allows us to look across the value chain. And we're looking at that in human health. But of course, if we get the right opportunity in animal health, we will look at that also. So, we will look at both. We have no clear definitive quantitative view as to what -- where that balance may end. And in terms of quantitative guidance on capacity utilization, again, we're not giving a number. We're looking to increase utilization of Mangalore through this year. In the first quarter, we've made a decent start. We're on track with where we wanted to be. It's in line with expectations, and that's a part of the reinforcement of our full year guidance.”
- Deepak Jain, CFO
Companies are making substantial CAPEX investments to expand capacity, upgrade technology, and build new capabilities, especially in high-growth areas.
Neuland Laboratories:
Cash outflow of INR 79 crores towards CAPEX this quarter. Expects commercial production from the additional production block in Unit 3 to start later this fiscal year. Peptide facility to be completed next financial year. Total CAPEX for FY26 estimated around INR 250 crores, with 60% for growth CAPEX and 40% for maintenance.
Jubilant Pharmova
Proposed investment of US$ 50 million in PET radiopharmacy network. Capacity expansion program in CDMO Sterile Injectables at Spokane is on track, with Line 3 expected to start commercial production in FY26. Total investment at Spokane is USD 285 Mn, with new lines having incremental revenue potential of USD 160 Mn to USD 180 Mn. Montreal facility also undergoing expansion (USD 114 million). Increasing capacity in CRDMO with total Capex USD 150 Mn, targeting 4,000 FTEs by FY30.
Laurus Labs
Q1 CAPEX reported at INR 265 Cr; 17% of Revenues. Ongoing strategic investments include groundbreaking of new Gene/ADC facility (Hyderabad) and Microbial fermentation facility (Vizag). Invested INR 215 Cr in KRKA joint venture (expected to be completed in mid-2027).
Blue Jet Healthcare
Increased CAPEX guidance for Unit-3 Mahad to INR 300 crores from INR 250 crores, with INR 200 crores to be incurred up to FY27. R&D center at Hyderabad at a cost of INR 40 crores. Plans to add another 1,000 KL capacity in the next two to three years by acquiring a large land parcel for a new CDMO site.
Hikal Limited
Q1 FY26 CAPEX was INR 31 crores, primarily for debottlenecking, regulatory upgrades, and CDMO capacity augmentation. Maintaining full-year CAPEX guidance of INR 200 crores.
OneSource Specialty Pharma: Accelerating Phase 2 of DDC capacity expansion to be operational a year ahead of schedule, still within the overall $100 million CAPEX plan. Also investing in new capabilities in sterile injectables and enhancing lyophilisation capacities.
Sai Life Sciences
Invested INR 134 crore in capital expenditure during Q1FY26. Commenced commercial operations at Bidar (PB-11 Phase II), adding ~91 KL capacity. Broke ground on a new Process R&D Block at Unit 2 Hyderabad, nearly doubling PRD capacity. Commencing work on building additional 200 KL production capacity at Unit IV, Bidar, by Q3 FY27. Strategic capital investments aligned with annual capex plan of ~ INR 700 Cr for FY26.
Divi's Laboratories
Anticipates total capital expenditure for FY26 to be in the range of INR 2,000 crores. Capitalized assets of INR 261 crores during Q1, including INR 114 crores for Phase 1 of Kakinada project.
There's a significant focus and investment in these advanced and high-growth areas, with companies actively building capabilities and capacity to capture market share.
Neuland Laboratories
"Our peptide investment plan is on track. We continue to garner more projects in this space, which further validates our excitement about the opportunities that the segment holds." - Saharsh Davuluri, Vice-Chairman and Managing Director. Notes 15+ years of experience in peptide chemistry as a differentiator. Expects new peptide facility to be completed next financial year.
TAM of Peptides opportunity grows led by GLP-1
Neuland has been dabbling with peptides for almost 17- 18 years now. We have had a small group, mostly working in small scale and then in pilot scale and now with this new investment, we wish to embark on large scale commercial manufacturing of peptides. The hypothesis for us has always been that, look, we are present in peptides. We understand the chemistry and the techniques of peptide chemistry. However, we never had large scale infrastructure. What has happened over the last several years, thanks to the GLP-1 is that peptides have become more prominent in the drug development landscape and peptides are being increasingly used as a vehicle for delivering new therapies. As a result, what used to be a $1 or $1.5 billion market space is now a $5 or $6 billion market space and it is growing very rapidly because more and more drugs are coming out into the market, which are peptides. As a result of this market expansion, a lot of players, not just globally but in India also are showing that enthusiasm and they are making investments in this space and they wish to participate in this opportunity. And I think it is a fairly large market and therefore I think there is room for several players. Having said that, I think what makes Neuland a strong player in the peptide space is the 15 plus years of experience we have had in peptide chemistry. If you look at the IP landscape, the number of patents filed by Neuland scientists over the years, the kind of work we have been doing, not just in terms of peptide APIs, but in peptide fragments, etc., I think that creates layers of depth of expertise that I believe Neuland uniquely has, at least from this region. In the space of peptides, techniques become that much more important than just knowledge of chemistry because when it comes to executing peptide projects, there are a lot of techniques, whether it is purification, lyophilization, precipitation, or different techniques, which are very difficult to achieve and apply to projects. Companies that have years of experience, especially even on the large scale, tend to do well and we believe that is what gives us the advantage. What was the limitation for Neuland, frankly speaking, was that we never had the large scale capacity. Even 4 or 5 years ago, we had been approached by biotech companies for making commercial peptides, but we could not fulfill those projects because we never had that scale. But now with the creation of this capacity, we are very confident that our business will grow, but I also do believe that there is enough space for multiple players to do well, but you do need strong R&D capabilities in peptides
-Saharsh Davuluri, Vice-Chairman and Managing Director
Jubilant Pharmova
Radiopharmaceuticals business is "on track to introduce multiple new products in the PET and SPECT imaging from FY27 to FY29." Dosing for Phase 2 clinical trial for MIBG is complete, with data package submission to FDA by H2’FY26.
Laurus Labs
Significant ongoing investment in Cell and Gene Therapy (CGT), with NexCAR19 showing continued demand. Groundbreaking on new dedicated Gene/Anti-body Drug Conjugates R&D and manufacturing facility in Hyderabad.
Blue Jet Healthcare
Building capacity to supply building blocks and peptide fragments to innovators and global CDMOs. Advancing plans to build a multipurpose plant at Mahad with focus on "newer chemistry platforms like peptides, intermediates for GLP-1s, biocatalysis." - VK Singh, COO. Developed about 45 peptide fragments.
Divi's Laboratories
"Notably, our newly commissioned Solid Phase Peptide Synthesis capacity has garnered strong interest from large pharmaceutical companies, particularly those developing GLP-1-based treatments."
- Kiran Divi, Whole-Time Director and CEO.
OneSource Specialty Pharma
New partnership with Xbrane in biologics "strengthens our drug device our sorry our drug substance business and accelerates also the the regulatory inspection of our drug substance site." - Neeraj Sharma, CEO & MD. Targeting GLP-1 commercialisation in H2 FY26. "our strength in one source is is drug device combinations... and products which where there is a interface between drug and device."
Syngene International
"A highlight for the quarter was the inauguration of the state-of-the-art dedicated peptide laboratory advancing our capability in this high potential growth area." - Peter Bains, Managing Director and CEO. Peptides complement existing capabilities in monoclonal antibodies, antibody-drug conjugates, and oligonucleotides. Unit 3 biologics facility operational, delivering a GMP clinical batch. Bayview Biologics facility in the US expected to operationalize in H2 FY26.
Maintaining high quality and compliance standards is a universal priority, with companies frequently reporting successful audits and proactive measures to address observations.
Neuland Laboratories:
"We continue to invest in our capabilities and people to ensure we are enhancing our customer experience, which we believe distinguishes us as a CDMO." - Saharsh Davuluri, Vice-Chairman and Managing Director. Unit 3 validations happening as per schedule.
Jubilant Pharmova
Montreal facility continued operations after successful implementation of corrective and preventive actions following an OAI classification.
Laurus Labs
Q1 FY26 saw 39 quality audits (39 customer, 0 regulatory). EIR status issued to Unit 4 API manufacturing facility.
Hikal Limited
The US FDA issued an OAI status to the Bangalore facility. "We want to assure all stakeholders that Hikal has taken this matter seriously and has already implemented comprehensive corrective and preventive actions in line with the agency's observations. These observations were procedural in nature and did not include any issues on data integrity." - Sameer Hiremath, Managing Director. Successfully concluded GMP audits by ANVISA (Brazil) and PMDA (Japan) at Bangalore API facility.
OneSource Specialty Pharma
Flagship site successfully cleared "back-to-back inspections from USFDA and ANVISA Brazil paving the way for commercialisation." - Neeraj Sharma, CEO & MD. Had almost 25 inspections done by regulatory agencies or customers across all sites, all successful.
Sai Life Sciences
"Successfully completed 11 client and regulatory audits across our sites further reinforcing our consistent track record of quality and compliance." - Krishna Kanumuri, MD & CEO.
Syngene International
Successfully completed a US FDA GCP inspection with no observations. The biologics facility at Biocon Park received an EIR with a favorable closure. Concluded over 20 client and regulatory audits.
China Plus One Strategy & Supply Chain Diversification:
This remains a significant tailwind for Indian CDMOs, driving increased RFPs and shifting manufacturing away from single-region dependencies.
Divi's Laboratories
"We are witnessing increased traction from global innovators who are actively seeking partners that can offer both scalability and reliability in a changing global supply chain landscape." - Kiran Divi, Whole-Time Director and CEO.
Hikal Limited
"CDMO business continues to benefit from the structural momentum driven by the China Plus One strategy." - Manoj Mehrotra, President, Pharmaceutical Business.
Jubilant Pharmova
Large innovator pharma companies are "looking to create an alternate manufacturing site in the US as a risk management strategy to mitigate any potential tariff’s imposed by the US govt." This is driving "excellent traction in Requests for Proposals (RFPs) for Line 3."
Blue Jet Healthcare
"We see structural tailwinds from de-risking of supply chains by global innovators."
- Shiven Arora, Managing Director.
Sai Life Sciences
"India Rising as a Strategic CRDMO Hub" driven by "Cost efficiency (30–40%) with global-standard quality" and "China-to-India outsourcing shift."
Syngene International
"China-independent supply chain development continued through strategic sourcing from the Indian ecosystem, and engagement with global suppliers to establish operations."
Mergers, Acquisitions, and Strategic Partnerships:
OneSource Specialty Pharma
Board of Directors evaluating potential acquisition of two USFDA-approved specialty injectable assets of Steriscience Specialities (Warsaw, Poland, and Vadodara, India). "Both these assets will add significant value to the long-term plans of one source which obviously will also mean that our targeted revenue of 400 million with 160 will now have an upward trajectory in excess of 500 million and in slightly in excess of $200 million a bit."
- Arun Kumar, Founder & Non-Executive Chairperson.
Customer Relationships and Pipeline Visibility:
Companies emphasize long-standing customer relationships and a healthy pipeline of projects across various development stages as key indicators of future growth.
Neuland Laboratories
The customer pipeline "gives us good visibility." - Abhijit Majumdar, CFO.
Divi's Laboratories
"Long-term trusted long-term partnerships we have cultivated with our customers over the years." - Kiran Divi, Whole-Time Director and CEO. Seeing "a healthy pipeline of RFPs and customer site visits along the side of multiple active projects progressing through R&D, pilot and validation stages."
Blue Jet Healthcare
"Long-standing relationships and multi-year contracts with multi-national customers." - Investor Presentation. Tracking about 20 new opportunities with high client interest, 30% in late phase III or commercial.
Syngene International
"We have continued to receive healthy interest from large and midsized pharma companies in the form of RFPs providing the basis for further growth in our research services in the coming quarters." - Peter Bains, Managing Director and CEO. Over 400 active clients, with 14 out of top 20 pharma companies as clients.
Macroeconomic & Geopolitical Uncertainties:
Global overcapacity, intensified price pressures, demand volatility, and tariff uncertainties continue to influence the external landscape.
Neuland Laboratories
"Given the rapidly changing environment, we continue to evaluate opportunities... While FY25 was a year of consolidation and Q1FY26 has been a continuation of that." - Abhijit Majumdar, CFO. "There are a variety of factors that could influence the projections. These include performance of individual products, foreign exchange fluctuations, raw material cost volatilities, and other dynamics of the business." - Saharsh Davuluri, Vice-Chairman and Managing Director.
Hikal Limited
"The chemical and life science sector continues to experience uneven recovery with pricing compression in select geographies, especially from Chinese competition. Tariff shifts and procurement variations due to trade realignments further complicated the external landscape leading to a muted first quarter." - Sameer Hiremath, Managing Director.
Divi's Laboratories
"The broader macro environments continue to be influenced by geopolitical uncertainties that are reshaping the global trade flows." - Nilima Motaparti, Whole-Time Director, Commercial. Pricing pressure on generics due to geopolitical situations and insurance companies cutting costs.
Syngene International
"Continued uncertainty in the biotech funding environment, which has not yet stabilized or returned to pre-pandemic levels." - Peter Bains, Managing Director and CEO.
Lumpiness of CDMO Business:
The inherent nature of the CDMO business, particularly with small-volume or early-stage products, can lead to uneven quarter-on-quarter or year-on-year performance.
Neuland Laboratories
"The inherent nature of our overall business is uneven on a quarter-on-quarter basis." - Abhijit Majumdar, CFO. "Evaluating Neuland's trajectory on a 3-year block basis is likely to be more accurate than comparing a QoQ or a YoY basis." - Saharsh Davuluri, Vice-Chairman and Managing Director.
Sai Life Sciences
"We would like to reiterate that the CDMO business by nature tends to be lumpy and is better reed on a longer-term basis." - Siva Chittor, CFO.
Blue Jet Healthcare
"for any B2B business, particularly a CDMO business, a quarter may not be a very good indication." - VK Singh, COO.
Regulatory Observations
Regulatory scrutiny remains high, and companies need to respond proactively and effectively to observations to maintain compliance and customer confidence.
Hikal Limited
OAI status from US FDA in February 2025 led to "temporary deferment of offtake in our pharmaceutical division." The company has "implemented comprehensive corrective and preventive actions" and is in "active communication with the U.S. FDA." - Sameer Hiremath, Managing Director.
The Indian Pharma CDMO and API sector, while facing short-term headwinds like global demand shifts and pricing pressures, stands on the cusp of significant long-term growth. The "China Plus One" strategy, increasing outsourcing by global innovators, and substantial investments in advanced capabilities like peptides and biologics are powerful tailwinds. Companies are proactively addressing challenges through strategic CAPEX, capacity expansion, and a relentless focus on quality and compliance, positioning themselves to capitalize on emerging opportunities. The Q1 FY26 results, though mixed, reflect a sector laying robust groundwork for a promising future.
Disclaimer: This blog post is for educational purposes only and should not be construed as a recommendation to buy or sell any securities. Investors should conduct their own due diligence and consult with a qualified financial advisor before making any investment decisions.
0 Comments